Service Objectives
The following list represents the Key Service Objectives (KSO) for the Appleton Greene Business Administration service.
Medical affairs
 The regulatory process can look very complex, whether your company is a multinational pharmaceutical or medical device company, or a company that is newly emerging in the marketplace. With proper planning and groundwork, successful submissions do not require last minute scrambles and overtime. Due to our extensive experience we can help you to deliver electronic submissions on time, on budget, and with less stress. Our Regulatory Affairs department are fully trained and experienced in preparing all major regulatory submissions for Phase II-III clinical trials, including complex multinational submissions for pharmaceutical products, biological products and medical devices. We prepare a full range of drug regulatory submissions for product licensing in Canada, USA and Europe: Abbreviated new drug applications (ANDA); Biologics license applications (BLA); Clinical trial authorization/application (CTA); Clinical trial notifications (CTN); Common technical document (CTD); Drug master files (DMF); Investigational device exemptions (IDE); Investigational new drug applications (INDA); Marketing authorization applications (MAA); New drug applications (NDA); New drug submissions (NDS); Orphan drug applications (ODA); Pre-market approvals (PMA).
The regulatory process can look very complex, whether your company is a multinational pharmaceutical or medical device company, or a company that is newly emerging in the marketplace. With proper planning and groundwork, successful submissions do not require last minute scrambles and overtime. Due to our extensive experience we can help you to deliver electronic submissions on time, on budget, and with less stress. Our Regulatory Affairs department are fully trained and experienced in preparing all major regulatory submissions for Phase II-III clinical trials, including complex multinational submissions for pharmaceutical products, biological products and medical devices. We prepare a full range of drug regulatory submissions for product licensing in Canada, USA and Europe: Abbreviated new drug applications (ANDA); Biologics license applications (BLA); Clinical trial authorization/application (CTA); Clinical trial notifications (CTN); Common technical document (CTD); Drug master files (DMF); Investigational device exemptions (IDE); Investigational new drug applications (INDA); Marketing authorization applications (MAA); New drug applications (NDA); New drug submissions (NDS); Orphan drug applications (ODA); Pre-market approvals (PMA).
Corporate change
 The linking of new leadership with attempted cultural change is not surprising when you consider that leadership and culture are essentially two sides of the same coin. A new CEO (or an existing one) can delegate many tasks, but the task of setting the culture cannot be delegated. Why? Because there is compelling evidence that business performance and culture are closely linked. If the CEO wants the business to achieve its corporate objectives, then the culture has to be right. In a major research program, Harvard professors John Kotter and James Heskett found consistent correlation between robust, engaged cultures and high-performance business results. Organisational culture is a problem when the way in which the organisation usually operates puts obstacles in the way towards achievement. Some examples: the goals of the organisation demand an external orientation of the members, but the organisational culture is characterized by internal orientation; transparency is needed to be accountable and to function democratically, but the tradition to involve family members and to favor them, may makes transparency cloudy; being value driven as a main characteristic of a NGO stands not well with the business-like attitude of our professionals; productivity, being directed towards goals, may be hindered by the grown habit to intervene in each other’s work, directing most of the energy to each other instead of to the product; the not-outspoken rule not to intervene in each other’s work (the non-intervention principle) may hinder the ideal working method of sharing ideas, innovations, solutions and problems; because of the one-sided fixation on ideology, satisfaction of personal needs may be considered as forbidden; a grown emphasis on output may have led to the situation in which reflection (base for learning) is considered a waste of time; organisational culture is not the result of just a decision, but the outcome of a lasting process, in which the attitude, beliefs and behavior of people are gradually shaped. Organisational culture, even if not objectively effective, is always a logical adaptation to a changed environment. Organisational culture may be compared to coping mechanisms: once effective in one specific situation, but internalized, unconscious familiar, and hardly noticeable for the owner. We will give you the processes and will guide you through the complex processes of corporate change and will make it a success.
The linking of new leadership with attempted cultural change is not surprising when you consider that leadership and culture are essentially two sides of the same coin. A new CEO (or an existing one) can delegate many tasks, but the task of setting the culture cannot be delegated. Why? Because there is compelling evidence that business performance and culture are closely linked. If the CEO wants the business to achieve its corporate objectives, then the culture has to be right. In a major research program, Harvard professors John Kotter and James Heskett found consistent correlation between robust, engaged cultures and high-performance business results. Organisational culture is a problem when the way in which the organisation usually operates puts obstacles in the way towards achievement. Some examples: the goals of the organisation demand an external orientation of the members, but the organisational culture is characterized by internal orientation; transparency is needed to be accountable and to function democratically, but the tradition to involve family members and to favor them, may makes transparency cloudy; being value driven as a main characteristic of a NGO stands not well with the business-like attitude of our professionals; productivity, being directed towards goals, may be hindered by the grown habit to intervene in each other’s work, directing most of the energy to each other instead of to the product; the not-outspoken rule not to intervene in each other’s work (the non-intervention principle) may hinder the ideal working method of sharing ideas, innovations, solutions and problems; because of the one-sided fixation on ideology, satisfaction of personal needs may be considered as forbidden; a grown emphasis on output may have led to the situation in which reflection (base for learning) is considered a waste of time; organisational culture is not the result of just a decision, but the outcome of a lasting process, in which the attitude, beliefs and behavior of people are gradually shaped. Organisational culture, even if not objectively effective, is always a logical adaptation to a changed environment. Organisational culture may be compared to coping mechanisms: once effective in one specific situation, but internalized, unconscious familiar, and hardly noticeable for the owner. We will give you the processes and will guide you through the complex processes of corporate change and will make it a success.
Regulatory compliance
 The regulatory process can look very complex, whether your company is a multinational pharmaceutical or medical device company or whether a company that is newly emerging in the marketplace. With proper planning and groundwork, successful submissions do not require last minute scrambles and overtime. Due to our extensive experience we can help you deliver electronic submissions on time, on budget, and with less stress. Having documentation accessible and organized for regulatory research and inspections for the clinical trials and then for the whole life of the product is one of the most important regulatory aspects. We help you through our specific and Customizable Document Management System (C-DMS) to have all documents filed and accessible all the time. For the clinical research sector this also opens us the possibility to create an electronic submission so that FDA reviewers receive them the day they are published. In addition we conduct critical appraisal and gap analysis, a technique for determining what useful information is missing in a submission and suggesting solutions for solving the problem of our clients’ submissions. Electronic submission services are available in addition to traditional paper document services.
The regulatory process can look very complex, whether your company is a multinational pharmaceutical or medical device company or whether a company that is newly emerging in the marketplace. With proper planning and groundwork, successful submissions do not require last minute scrambles and overtime. Due to our extensive experience we can help you deliver electronic submissions on time, on budget, and with less stress. Having documentation accessible and organized for regulatory research and inspections for the clinical trials and then for the whole life of the product is one of the most important regulatory aspects. We help you through our specific and Customizable Document Management System (C-DMS) to have all documents filed and accessible all the time. For the clinical research sector this also opens us the possibility to create an electronic submission so that FDA reviewers receive them the day they are published. In addition we conduct critical appraisal and gap analysis, a technique for determining what useful information is missing in a submission and suggesting solutions for solving the problem of our clients’ submissions. Electronic submission services are available in addition to traditional paper document services.
Project management
 For each project, we select and assign Project Managers with relevant experience that corresponds to client’s requirements and specifications. We, along with our clients, gather necessary trial-specific information, analyse the project goals, assess risks, plan, run the project simulation, identify any gaps and then execute the project. During the preparation process, client’s team is able to get to know our candidates for Project Management role and identify the most suitable person. Since we know that the key to a successful trial is project management, our Project Managers “own” their projects and are fully accountable for the outcomes. They are provided and use the state-of-the-art technology to be in real time communication with the sites, the client and the study team in order to maintain control over the project. Our Project Managers have to respond quickly to the client’s needs, to anticipate possible problems and to provide the best solution available. We provide the project management that the trial needs and the relationship to the client that is needed to succeed. The Global Project Manager will have the following responsibilities: Ensure the study complies with all regulatory guidelines; Participates in all stages of project development – planning, execution and close out; Orchestrates the project team performance to achieve required efficiency and cohesiveness; Serves as a key liaison between the project team, Client and external vendors; Keeps the client updated on the project developments; Offers a flexible proactive approach to resolve issues promptly and effectively; Provide weekly reports of the project including identification of potential risks and contingency plans; Ensures consistency in quality and procedures; Becomes part of the client’s decision process by offering proactive suggestions and solutions; Assesses emerging issues and resolves them according to the project-specific documents; escalates the issues to senior management and client as necessary; Ensures the project deadlines/ deliverables are met; Manages and owns the project; Manages the budget and provides financial managerial reports as necessary. Working with us provides many advantages: Our PM are equally or more experienced than those from larger companies and the turnover rate is lower; Our PMs allow clients to maintain control over decisions to a level of control where larger companies may take total control over the project; We provide more value and ROI due to lower operational costs; We focus on each project as if it would be our own as we want the project to succeed and the client to be fully satisfied. We want our clients to return to us; We provide a higher level of trial detail because we know that this detail is critical to the success of the project and this could be a life and death situation for smaller clients.
For each project, we select and assign Project Managers with relevant experience that corresponds to client’s requirements and specifications. We, along with our clients, gather necessary trial-specific information, analyse the project goals, assess risks, plan, run the project simulation, identify any gaps and then execute the project. During the preparation process, client’s team is able to get to know our candidates for Project Management role and identify the most suitable person. Since we know that the key to a successful trial is project management, our Project Managers “own” their projects and are fully accountable for the outcomes. They are provided and use the state-of-the-art technology to be in real time communication with the sites, the client and the study team in order to maintain control over the project. Our Project Managers have to respond quickly to the client’s needs, to anticipate possible problems and to provide the best solution available. We provide the project management that the trial needs and the relationship to the client that is needed to succeed. The Global Project Manager will have the following responsibilities: Ensure the study complies with all regulatory guidelines; Participates in all stages of project development – planning, execution and close out; Orchestrates the project team performance to achieve required efficiency and cohesiveness; Serves as a key liaison between the project team, Client and external vendors; Keeps the client updated on the project developments; Offers a flexible proactive approach to resolve issues promptly and effectively; Provide weekly reports of the project including identification of potential risks and contingency plans; Ensures consistency in quality and procedures; Becomes part of the client’s decision process by offering proactive suggestions and solutions; Assesses emerging issues and resolves them according to the project-specific documents; escalates the issues to senior management and client as necessary; Ensures the project deadlines/ deliverables are met; Manages and owns the project; Manages the budget and provides financial managerial reports as necessary. Working with us provides many advantages: Our PM are equally or more experienced than those from larger companies and the turnover rate is lower; Our PMs allow clients to maintain control over decisions to a level of control where larger companies may take total control over the project; We provide more value and ROI due to lower operational costs; We focus on each project as if it would be our own as we want the project to succeed and the client to be fully satisfied. We want our clients to return to us; We provide a higher level of trial detail because we know that this detail is critical to the success of the project and this could be a life and death situation for smaller clients.
Quality assurance
 Quality assurance and compliance is mandatory in order to ensure the integrity of the clinical trials. Our experienced GCQA department helps our clients to verify the integrity of the scientific data and adhere to the protocols and to the regulatory requirements. Our GCQA staff is knowledgeable about the local, national and international regulations and guidelines. Our GCQA team contains also physicians and legal experts with GCQA experience. We provide a full range of GCP Quality Assurance and Compliance services.
Quality assurance and compliance is mandatory in order to ensure the integrity of the clinical trials. Our experienced GCQA department helps our clients to verify the integrity of the scientific data and adhere to the protocols and to the regulatory requirements. Our GCQA staff is knowledgeable about the local, national and international regulations and guidelines. Our GCQA team contains also physicians and legal experts with GCQA experience. We provide a full range of GCP Quality Assurance and Compliance services.
Biotechnology
Biotechnology is the use of living systems and organisms to develop or make useful products, or any technological application that uses biological systems, living organisms or derivatives thereof, to make or modify products or processes for specific use. For thousands of years, humankind has used biotechnology in agriculture, food production, and medicine. Biotechnology has applications in four major industrial areas, including health care (medical), crop production and agriculture, non food (industrial) uses of crops and other products (e.g. biodegradable plastics, vegetable oil, bio-fuels), and environmental uses. The biotechnology market consists of the development, manufacturing, and marketing of products based on advanced biotechnology research. The global biotechnology market has total revenues of $304.0bn, representing a compound annual growth rate (CAGR) of 9.6%. The medical/healthcare segment is the market’s most lucrative, with total revenues of $182.5bn, equivalent to 60.0% of the market’s overall value. The performance of the market is forecast to decelerate, with an anticipated CAGR of 9%, which is expected to drive the market to a value of $468.2bn.
Consultancy
Management consulting, the practice of helping organizations to improve their performance, operates primarily through the analysis of existing organizational problems and the development of plans for improvement. Organizations may draw upon the services of management consultants for a number of reasons, including gaining external (and presumably objective) advice and access to the consultants’ specialized expertise. Consultancies may also provide organizational change-management assistance, development of coaching skills, process analysis, technology implementation, strategy development, or operational improvement services. Management consultants often bring their own proprietary methodologies or frameworks to guide the identification of problems and to serve as the basis for recommendations for more effective or efficient ways of performing work tasks. Management consulting has grown quickly, with growth rates of the industry exceeding 20% during the past 30 years. As a business service, consulting remains highly cyclical and linked to overall economic conditions. Currently, there are three main types of consulting firms. Large, diversified organizations, Medium-sized management consultancies and boutique firms that have focused areas of consulting expertise in specific industries, functional areas, technologies, or regions of the world. The value of the management & marketing consultancy market is calculated as the total revenues received for the provision of corporate strategy services, operations management services, information technology solutions, human resource management services and outsourcing services. The global management & marketing consultancy market has total revenues of $305.0bn, representing a compound annual growth rate (CAGR) of 3%. The operations management segment is the market’s most lucrative, with total revenues of $93bn, equivalent to 30.5% of the market’s overall value. The performance of the market is forecast to accelerate, with an anticipated CAGR of 7% during the next 5 years, which is expected to drive the market to a value of $427.9bn.
Government
The economic, financial and military pressures on global governments are especially high in today’s world. Those that perform best under pressure are armed with insight that helps identify new or missed tax revenue opportunities, reduce fraud and waste in human health services, effectively manage key military assets, and analyze and predict events related to security intelligence. From state and local issues – to national security at home and abroad, all levels of government are faced with the daunting task of collecting and analyzing data and assuring compliance, accurately and in real time.
Healthcare
The health care industry, or medical industry, is an aggregation of sectors within the economic system that provides goods and services to treat patients with curative, preventive, rehabilitative, and palliative care. The modern health care industry is divided into many sectors and depends on interdisciplinary teams of trained professionals and paraprofessionals to meet health needs of individuals and populations. The health care industry is one of the world’s largest and fastest-growing industries. Consuming over 10 percent of gross domestic product (GDP) of most developed nations, health care can form an enormous part of a country’s economy. For purpose of finance and management, the health care industry is typically divided into several areas. As a basic framework for defining the sector, the United Nations International Standard Industrial Classification (ISIC) categorizes the health care industry as generally consisting of: hospital activities; medical and dental practice activities; “other human health activities”. This third class involves activities of, or under the supervision of, nurses, midwives, physiotherapists, scientific or diagnostic laboratories, pathology clinics, residential health facilities, or other allied health professions, e.g. in the field of optometry, hydrotherapy, medical massage, yoga therapy, music therapy, occupational therapy, speech therapy, chiropody, homeopathy, chiropractics, acupuncture, etc. The Global Industry Classification Standard and the Industry Classification Benchmark further distinguish the industry as two main groups: health care equipment and services; and pharmaceuticals, biotechnology and related life sciences. Health care equipment and services comprise companies and entities that provide medical equipment, medical supplies, and health care services, such as hospitals, home health care providers, and nursing homes. The second industry group comprises sectors companies that produce biotechnology, pharmaceuticals, and miscellaneous scientific services. Other approaches to defining the scope of the health care industry tend to adopt a broader definition, also including other key actions related to health, such as education and training of health professionals, regulation and management of health services delivery, provision of traditional and complementary medicines, and administration of health insurance. The global medical device industry has experienced significant growth over the last five years and is expected to continue, reaching approximately US $302 billion with a CAGR of 6.1% during the next five years. The medical device industry is comprised of surgical, cardiovascular, home healthcare, general medical and other devices. The industry is highly fragmented, and North America dominates with 46% of the global market. High competitive rivalry prevails with low to moderate barrier for entry into the industry. The aging population and growing demand for convenient and cost-effectiveness products are expected to drive the global home healthcare device industry, and the home healthcare device market is expected to reach an estimated US $29 billion with a CAGR of 3.4% over the next five years. The home healthcare device industry consists of home-based treatment such as glucose monitor, blood pressure monitor, diabetic control device, wheelchair, walking aids, oxygen inhaler, thermometer, home dialysis, test strips, heart rate meters, sleep monitor device, and such other home healthcare devices. A combination of factors such as technological innovations, aging population, rising patient pool, and changing lifestyle is seen to impact the market dynamics significantly.
Pharmaceutical
The pharmaceutical industry develops, produces, and markets drugs or pharmaceuticals licensed for use as medications. Pharmaceutical companies are allowed to deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations regarding the patenting, testing and ensuring safety and efficacy and marketing of drugs. Drug companies are like other companies in that they manufacture products that must be sold for a profit in order for the company to survive and grow. They are different from some companies because the drug business is very risky. For instance, only one out of every ten thousand discovered compounds actually becomes an approved drug for sale. Much expense is incurred in the early phases of development of compounds that will not become approved drugs. In addition, it takes about 7 to 10 years and only 3 out of every 20 approved drugs bring in sufficient revenue to cover their developmental costs, and only 1 out of every 3 approved drugs generates enough money to cover the development costs of previous failures. This means that for a drug company to survive, it needs to discover a blockbuster (billion-dollar drug) every few years. Industry-wide research and investment has reached a record $65.3 billion. While the cost of research in the U.S. is about $34.2 billion, revenues rose by $200.4 billion. A study by the consulting firm Bain & Company reported that the cost for discovering, developing and launching (which factored in marketing and other business expenses) a new drug (along with the prospective drugs that fail) rose over the last five years to nearly $1.7 billion. According to Forbes, development costs are between $4 billion to $11 billion per drug. The United States accounts for more than a third of the global pharmaceutical market, with $340 billion in annual sales followed by the EU and Japan. Emerging markets such as China, Russia, South Korea and Mexico outpaced that market, growing a huge 81 percent. According to IMS the global pharmaceutical industry is estimated at US$1.1 trillion.
Bronze Service

Monthly cost: USD $1,500.00
Time limit: 5 hours per month
Contract period: 12 months
Bronze service includes:
01. Email support
02. Telephone support
03. Questions & answers
04. Professional advice
05. Communication management

SERVICE DESCRIPTION
The Bronze Client Service (BCS) for Business Administration provides clients with an entry level option and enables client contacts to become personally acquainted with Dr. Popa over a sustainable period of time. We suggest that clients allocate up to a maximum of 5 Key Employees for this service. Your Key Employees can then contact the consultant via email, whenever they feel that they need specific advice or support in relation to the consultant’s specialist subject. The consultant will also be proactive about opening and maintaining communications with your Key Employees. Your Key Employees can list and number any questions that they would like to ask and they will then receive specific answers to each and every query that they may have. Your Key Employees can then retain these communications on file for future reference. General support inquiries will usually receive replies within 48 hours, but please allow a period of up to 10 business days during busy periods. The Bronze Client Service (BCS) enables your Key Employees to get to know their designated Appleton Greene consultant and to benefit from the consultant’s specialist skills, knowledge and experience.
Silver Service

Monthly cost: USD $3,000.00
Time limit: 10 hours per month
Contract period: 12 months
Bronze service plus
01. Research analysis
02. Management analysis
03. Performance analysis
04. Business process analysis
05. Training analysis

SERVICE DESCRIPTION
The Silver Client Service (SCS) for Business Administration provides more time for research and development. If you require Dr. Popa to undertake research on your behalf, or on behalf of your Key Employees, then this would understandably require more time and the Silver Client Service (SCS) accommodates this. For example, you may want your consultant to undertake some research into your management, performance, business, or training processes, with a view towards providing an independent analysis and recommendations for improvement. If any research and development, or business analysis is required, then the Silver Client Service (SCS) is for you.
Gold Service

Monthly cost: USD $4,500.00
Time limit: 15 hours per month
Contract period: 12 months
Bronze/Silver service plus
01. Management interviews
02. Evaluation and assessment
03. Performance improvement
04. Business process improvement
05. Management training

SERVICE DESCRIPTION
The Gold Client Service (GCS) for Business Administration is intended for more detailed evaluation and assessment, that may require your Key Employees to have monthly meetings or interviews with Dr. Popa. These meetings and interviews can be conducted over the telephone, Skype, or by video conference if required. The consultant can also attend your business premises, an Appleton Greene office, or another mutually beneficial location, but please note that clients are responsible for the costs of any disbursements separately, including travel and accommodation. This service enables you to integrate the specific skills, knowledge and experience of your designated consultant into your Key Employee management team. The Gold Client Service (GCS) can also incorporate training workshops, business presentations and external meetings with customers, suppliers, associations, or any other business-related stakeholders.
Platinum Service

Monthly cost: USD $6,000.00
Time limit: 20 hours per month
Contract period: 12 months
Bronze/Silver/Gold service plus
01. Project planning
02. Project development
03. Project implementation
04. Project management
05. Project review

SERVICE DESCRIPTION
The Platinum Client Service (PCS) for Business Administration is our flagship service and will be required if you need Dr. Popa to facilitate the planning, development, implementation, management, or review of a particular project relating to his specialist subject, which would obviously require more time and dedication. This service enables you to reserve up to 12.5% of the consultant’s working month and provides a more hands-on service as and when required. If you need more time than this, then this can always be arranged, subject of course to the consultant’s ongoing availability. The benefit of having an external consultant involved in projects is they provide an independent perspective and are not influenced by internal politics, day-to-day responsibilities, or personal career interest. They provide objectivity, specific knowledge, skills and experience and will be entirely focused upon the tasks at hand. The Platinum Client Service (PCS) will provide your organization with a valuable resource as and when you need it.
Benefits
Management
- Performance management
- Improved efficiency
- Customer satisfaction
- Improved effectiveness
- Improved growth
- Improved competitiveness
- Better flexibility
- Decreased risk
- Increased quality
- More opportunities
Information Technology
- Accurate processing
- Quick processing
- Globalization
- Effective communication
- Improved efficiency
- Improved effectiveness
- Remote accessibility
- Performance evaluation
- Data storage
- Increased security
Marketing
- Improved communication
- Decreased risk
- Improved reputation
- Market data
- Improved planning
- Better management
- Establish trends
- Market positioning
- Increased profit
- Customer loyalty
Clients
This service’s current clients or employers include:

Amgen
Amgen is committed to unlocking the potential of biology for patients suffering from serious illnesses by discovering, developing, manufacturing and delivering innovative human therapeutics. This approach begins by using tools like advanced human genetics to unravel the complexities of disease and understand the fundamentals of human biology. Amgen focuses on areas of high unmet medical need and leverages its biologics manufacturing expertise to strive for solutions that improve health outcomes and dramatically improve people’s lives. A biotechnology pioneer since 1980, Amgen has grown to be one of the world’s leading independent biotechnology companies, has reached millions of patients around the world and is developing a pipeline of medicines with breakaway potential. Amgen’s medicines treat serious illnesses. We have a presence in more than 75 countries worldwide and have reached millions of people in the fight against cancer, kidney disease, rheumatoid arthritis, bone disease and other serious illnesses. Our medicines typically address diseases for which the number of effective treatment options is limited, or they are medicines that provide a viable option to what is otherwise available. Understanding the fundamental biological mechanisms of human life is a defining feature of Amgen’s discovery research efforts—and a major contributor to the development of Amgen’s deep and broad pipeline of potential new medicines. Amgen’s “biology first” approach permits its scientists to first explore the complex molecular pathways of disease before determining what type of medicine, or modality, is most likely to deliver optimal efficacy and safety. As advances in human genetics continue to shed new light on the molecular roots of disease, we expect to continue to improve how we identify and validate human disease targets. The treatment of millions of seriously ill patients worldwide depends on the safe and reliable production of biologic medicines, which are administered by injection or intravenously. A worldwide leader in biologics manufacturing, Amgen has an outstanding track record of reliably delivering high-quality medicines to patients who need them. Significant skill, experience, vigilance and commitment are critical to help ensure the quality of a biologic medicine each time a new batch is made. At Amgen, robust quality control and a reliable supply of medicines for patients are every bit as important as scientific innovation.

Bayer
Bayer is a global enterprise with core competencies in the Life Science fields of health care and agriculture. Its products and services are designed to benefit people and improve their quality of life. At the same time, the Group aims to create value through innovation, growth and high earning power. Bayer is committed to the principles of sustainable development and to its social and ethical responsibilities as a corporate citizen. For over 150 years Bayer has been an innovation company rooted in science. Our presence in the U.S. harkens back to the mid-19th century as a chemical company making dyes in Albany, NY. However, by the late-19th century we moved into the healthcare field with the introduction of a number of medicines including our most well-known product, Aspirin. Today, we continue striving to meet the world’s challenges through scientific research, persistence, and courageous innovation. At Bayer, innovation begins, as it always has, with our products. In line with our mission “Bayer: Science For A Better Life,” we aim to improve people’s quality of life. For this endeavour, we focus on our core competency of developing and successfully commercializing innovative products and solutions based on scientific knowledge. We are improving people’s quality of life by preventing, alleviating or curing diseases. We are developing innovative care for animals – providing family pets with a longer, better quality of life and ensuring the health and safety of livestock. Our solutions help farmers in America provide adequate nutrition to our growing population while maintaining the health and sustainability of our environment. Our high-tech polymer materials are making significant contributions in areas such as energy and resource efficiency for mobility, construction and home living. We believe that passionate people have the power to create change. Our business success is largely attributable to the knowledge, skills and commitment of our employees. It is their ability to innovate and their willingness to embrace continuous development that drives our position as a world-class innovation company. Sustained success is only possible if we create working conditions that allow all employees to utilize their talents optimally. This is why we value diverse perspectives and provide our employees with the ongoing training they need to remain at the top of their respective fields. Our programs support a better life – today and into tomorrow. Our long-term commitment to science for a better life led Bayer to develop its award-winning Bayer Making Science Make Sense (MSMS) program. For 20 years, MSMS has advanced science literacy across the United States through hands-on, inquiry-based science learning, and employee volunteerism. STEM (science, technology, engineering, mathematics) education, particularly targeted to women and minorities who are under-represented in STEM-jobs, will continue to be vital to our company’s ability to preserve human, animal and nutritional health in the future. By educating and engaging the youth of today in STEM, we ensure that Bayer will continue to benefit society in innovative new ways for generations to come.

INC Research
INC Research is a leading, global full-service clinical research organization (CRO) providing the full range of Phase I to IV clinical development services for the world’s pharmaceutical, biotech and medical device industries. As a therapeutically focused CRO, with a Trusted Process delivery methodology, developing the medicines people need is something we take personally. Leveraging the breadth of our service offerings and the depth of our therapeutic expertise across multiple patient populations, INC Research connects customers, investigative sites and patients to accelerate the delivery of new medicines to market to improve world health. We were ranked “Top CRO to Work With” by sites worldwide in the 2015 Center Watch Global Investigative Site Relationship Survey. INC Research is headquartered in Raleigh, NC, with operations across six continents and experience spanning more than 100 countries.

Lilly
We were founded in 1876 by Colonel Eli Lilly, a man committed to creating high-quality medicines that meet real needs. More than a century later, we are passionate about building on this precedent in our continued pursuit to make life better for individuals, communities, and the world around us. Our heritage and our values are the foundation of our promise to unite caring with discovery to make life better for people around the world. Our mission: We make medicines that help people live longer, healthier, more active lives. Our values: Integrity, excellence, respect for people. Our vision: We will make a significant contribution to humanity by improving global health in the 21st century. Lily at a glance: A heritage 139 years strong: company founded on May 10, 1876; Headquarters located in Indianapolis, Indiana, U.S.A; Approximately 41,000 employees worldwide; More than 8,000 employees engaged in research and development; Clinical research conducted in more than 55 countries; Research and development facilities located in six countries; Manufacturing plants located in 13 countries; Products marketed in 120 countries.

Medivation
Medivation blends a unique business model with an expert team to bring promising medical technologies from the lab bench to the patient bedside. Our enzalutamide clinical development program addresses a large market with significant unmet medical needs. Medivation was founded by a group of experienced professionals with a long track record of working together successfully as a team in the pharmaceutical, biotechnology and medical device industries. We founded Medivation to leverage that expertise to bridge the medical product development gap between early-stage development and product launch. Through our extensive network of contacts with top-flight scientists and research institutions, we acquire early-development stage pharmaceuticals and medical devices that have promising clinical, intellectual property and commercial prospects. Using the extensive development experience and expertise of our core team, supplemented by expert consultants in relevant functions, we identify and execute the strategic pathway that will allow the most rapid, efficient and effective development. Our business strategy is straightforward: Build a portfolio of four to six product candidates that have the potential to be in clinical development within 12 to 18 months after acquisition; Develop those product candidates as rapidly and efficiently as possible; and Consider partnering or selling successful programs to large pharmaceutical, biotechnology or medical device companies for late-stage clinical studies and commercialization. In October 2009, Medivation and Astellas entered into a global agreement to jointly develop and commercialize enzalutamide. The companies are collaborating on a comprehensive development program that includes studies to develop enzalutamide across the full spectrum of advanced prostate cancer as well as advanced breast cancer. The companies jointly commercialize XTANDI in the U.S. and Astellas has responsibility for manufacturing and all additional regulatory filings globally, as well as commercializing XTANDI outside the U.S.
Locations
This service is primarily available within the following locations:

Montreal QC
Montreal is the city with the second-largest economy among Canadian cities based on GDP and the largest in Quebec. The city is an important center of commerce, finance, industry, technology, culture, world affairs and its main industries include aerospace, electronic goods, pharmaceuticals, printed goods, software engineering and telecommunications,. The service sector is also highly developed and includes civil, mechanical and process engineering, finance, higher education, and research and development. The city hosts a major international airport which is also a major hub for Air Canada. The Port of Montreal is the largest inland port in the world handling 26 million tons of cargo annually. As one of the most important ports in Canada, it remains a trans-shipment point for grain, sugar, petroleum products, machinery, and consumer goods. For this reason, Montreal is the railway hub of Canada and has always been an extremely important rail city; it is home to the headquarters of the Canadian National Railway, and was home to the headquarters of the Canadian Pacific. Montreal saw a huge development in the last 15 years. Having one of the most modern business laws and due to important incentives from provincial government and highly qualified workforce, the city attracted a lot of investments in the last period of time. Major companies from all industries are represented in Montreal. It is expected for the next years that the positive trend continues, making Montreal a very attractive destination for investors and companies. Montreal should be a location for any company willing to do business in Canada.

Toronto ON
Toronto is an international centre for business and finance, being considered the financial capital of Canada. The Toronto Stock Exchange is the world’s seventh-largest stock exchange by market capitalization. The five largest financial institutions of Canada, collectively known as the Big Five, have national offices in Toronto. The city is an important centre for the media, publishing, telecommunication, information technology and film production industries; it is home to Bell Media, Rogers Communications, and Torstar. Although much of the region’s manufacturing activities take place outside the city limits, Toronto continues to be a wholesale and distribution point for the industrial sector. The city hosts a major international airport which hosts Air Canada. More than 30 million travellers visit Toronto Pearson International Airport yearly. Although it doesn’t host its own port, the city’s strategic position along the Quebec City – Windsor Corridor and its road and rail connections help support the nearby production of motor vehicles, iron, steel, food, machinery, chemicals and paper. The completion of the Saint Lawrence Seaway gave ships access to the Great Lakes from the Atlantic Ocean. For this reason, Toronto is a railway hub of Canada and has always been an extremely important rail city. Toronto saw a huge development in the last years. It continues to attract a lot of Canadian and international investors and companies that want to do business in Canada. It is expected for the next years that the positive trend continues, making the city a very attractive destination for investors and companies. Toronto is a must location for any company willing to do business in Canada.

London UK
London generates approximately 20 per cent of the UK’s GDP (or $446 billion); while the economy of the London metropolitan area – the largest in Europe – generates approximately 30 per cent of the UK’s GDP (or an estimated $669 billion). London is a leading global city, with strengths in the arts, commerce, education, entertainment, fashion, finance, healthcare, media, professional services, research and development, tourism, and transport all contributing to its prominence. It is one of the pre-eminent financial centers of the world and is recognized to be, together with New York City, as the most important location for international finance. London is a world cultural capital. It is the world’s most-visited city as measured by international arrivals and has the world’s largest city airport system measured by passenger traffic. London is the world’s leading investment destination, hosting more international retailers and ultra-high-net-worth individuals than any other city. London’s 43 universities form the largest concentration of higher education institutes in Europe, and a 2014 report placed it first in the world university rankings. According to the report London also ranks first in the world in software, multimedia development and design, and shares first position in technology readiness. The City of London is home to the Bank of England, London Stock Exchange, and Lloyd’s of London insurance market. Over half of the UK’s top 100 listed companies (the FTSE 100) and over 100 of Europe’s 500 largest companies have their headquarters in central London. Over 70 per cent of the FTSE 100 are within London’s metropolitan area, and 75% of Fortune 500 companies have offices in London.

Boston MA
A global city, Boston is placed among the top 30 most economically powerful cities in the world. Encompassing $363 billion, the Greater Boston metropolitan area has the sixth-largest economy in USA and 12th-largest in the world. Boston’s colleges and universities have a significant effect on the regional economy. Boston attracts more than 350,000 college students from around the world, who contribute more than $4.8 billion annually to the city’s economy. The area’s schools are major employers and attract industries to the city and surrounding region. The city is home to a number of technology companies and is a hub for biotechnology, with the Milken Institute rating Boston as the top life sciences cluster in the USA. The city is also considered highly innovative for a variety of reasons that include the presence of academia, access to venture capital, and the presence of many high-tech companies. Because of Boston’s status as a state capital and the regional home of federal agencies, law and government are another major component of the city’s economy. The city is a major seaport along the United States’ East Coast and the oldest continuously operated industrial and fishing port in the Western Hemisphere. Other important industries are financial services, especially mutual funds and insurance. The city is a center for venture capital firms. Boston is a printing and publishing center. The city is home to three major convention centers—the Hynes Convention Center in the Back Bay, and the Seaport World Trade Center and Boston Convention and Exhibition Center. Several major companies headquartered within Boston or nearby—especially along Route 128, the center of the region’s high-tech industry. In 2006 Boston and its metropolitan area ranked as the fourth-largest cyber-city in the United States with 191,700 high-tech jobs.
Testimonials

Bayer HealthCare
“I’ve worked with Mr. Popa for the last 2 years on a very hi profile project for the company. He is very professional and knowledgeable. He also has great relationships with his sites and investigators and he is a very important member of the team. I highly recommend Mr. Popa for his work. Mr. Popa is a well-known expert whose experience, medical and regulatory knowledge combined with his extensive medical and business background helped pharmaceutical and biotech companies to complete successful clinical trials and successfully register compounds in the North American market. Mr. Popa started in the industry in Europe in 1996 as Medical representative and climbed the ranks to a top management position in Romania. Guided by this principles, Mr. Popa has led companies to breakthrough results, highlighted by the following: Strategy Management discussion with the study team and establishing a strategy for product development (number and types of clinical trials, timeframe, etc); Reduced Project Costs lowered the number of and eliminated unnecessary project procedures, improved operational processes; Improvement of Project Quality and Compliance training of project team for audits; development of customized trainings for different project participants; Improving Project Timelines improving efficiency and efficacy of processes, the building of highly experienced and efficient project team, improvement of project procedures and operational aspects of the study. Dr. Popa has consulted for many companies like Amgen, Imclone, Lilly, Medivation, Abbott, Optimer Pharma and Bayer for pivotal projects. His solid business, clinical research, quality assurance and medical background together with his broad international experience helped him to run successfully a full range of projects. He is a polyglot, being fluent in English, French, German and Romanian. As a dynamic public speaker, Mr. Popa is a presenter at investigator’s meetings, provides public speaking for various companies in the field of clinical research. Due to his tenure, he was named by Strathmore’s Who’s Who and American Registry for Outstanding Professionals as “Professional of the year 2014 in Clinical Research.”

INC Research
“Mr. Popa is one of the most competent clinical research professionals I have ever met. He knows and utilizes the principles of ICH and GCPs in all of his work. Mr. Popa’s site management ability is excellent; his sites love working with him and he is able to get results very easily. Mr. Popa is every project manager’s dream! Mr. Popa is an extremely hardworking and dedicated team player. He is always willing to assist with any task to meet project timelines. He has also brought a wealth of skills in many areas that proved to be significant assets to the team as a whole. His medical background along with in-depth understanding of clinical research as a whole only enhances his abilities to handle any task that is given to him. It was a true pleasure to work with him and I hope to have the opportunity to work with him in the future. Mr. Popa would most definitely be a tremendous asset to any organization.”
More detailed achievements, references and testimonials are confidentially available to clients upon request.
Personal Profile
Mr Chicles is an approved Certified Learning Provider (CLP) at Appleton Greene who is a business leader and strategist with broad experience in the global multi-industrial, aerospace and defense sectors. He is a seasoned operational leader of global industrial businesses, leading transformational strategies in highly competitive markets.
As a senior, C-suite strategist for multiple major industrial corporations he has led multiple mergers, acquisitions, divestitures and restructurings, as well as corporate break-ups and spin-offs. He has a distinguished track record of successful transformations of complex organizations in dynamic and uncertain market conditions while engendering the trust and buy-in of employees, customers, vendors, owners, corporate leadership and boards of directors.
A highly engaged leader at the personal and team level he has demonstrated the ability to engender effective senior teams and boards. He’s also an active mentor, teacher and community leader.
Mr Chicles is an active board member with AES Seals, global leader in sustainable reliability engineering, and Micro Technologies Inc, an electronics and advanced manufacturing company. He is a principal partner with ProOrbis Enterprises®, a management science consultancy with premier clients such as the US Navy and PwC, as well as the principal of Xiphos Associates™, a management and M&A advisory. Recently, he served as Board Director and Chairman of Global Business Development with Hydro Inc. the largest independent pump and flow systems engineering services provider in the world.
He was President of ITT’s Industrial Process / Goulds Pumps business segment a global manufacturer of industrial pumps, valves, monitoring and control systems, and aftermarket services for numerous industries with $1.2 billion in revenue, 3,500 employees and 34 facilities in 17 countries. Preceding this role he served as Executive Vice President of ITT Corporation overseeing the creation of a newly conceived ITT Inc. following the break-up of the former ITT Corporation to establish its strategy and corporate functions such as HR, communications, IT and M&A, building the capabilities, policies and organizations for each.
He joined ITT Corporation’s executive committee as its strategy chief in 2006 and instituted disciplined strategic planning processes and developed robust acquisition pipelines to respond to rapidly changing markets. Created successful spin-offs of 2 new public corporations Exelis Inc. and Xylem Inc. ITT Corporation was named one of “America’s Most Respected Corporations” by Forbes for exemplary management and performance during his tenure there.
Before joining ITT, Mr Chicles served as Vice President of Corporate Business Development and head of mergers and acquisitions for American Standard / Trane Companies, where he initiated and closed numerous transactions and equity restructurings globally.
Additionally, he created and led the corporate real estate function which entailed more than 275 real estate transactions around the world.
He began his career at Owens Corning rising through the ranks in various operational roles to Vice President of Corporate Development.
Recently, he taught advanced enterprise strategy at Stevens Institute of Technology as an adjunct professor and still supports start-ups through the Stevens Venture Center. He continues to be active as the Founding Board Member with several successful start-up technology businesses and non-profit organizations. A community leader, Mr Chicles has held the role of President of the Greek Orthodox Cathedral in Tenafly, N.J., He also led trips abroad to Cambodia and Costa Rica to build sustainable clean-water solutions and affordable housing.
His formal education includes earning a Masters of Business Administration from The Wharton School at the University of Pennsylvania, and a Bachelors in Finance from Miami University.
(CLP) Programs

Appleton Greene corporate training programs are all process-driven. They are used as vehicles to implement tangible business processes within clients’ organizations, together with training, support and facilitation during the use of these processes. Corporate training programs are therefore implemented over a sustainable period of time, that is to say, between 1 year (incorporating 12 monthly workshops), and 4 years (incorporating 48 monthly workshops). Your program information guide will specify how long each program takes to complete. Each monthly workshop takes 6 hours to implement and can be undertaken either on the client’s premises, an Appleton Greene serviced office, or online via the internet. This enables clients to implement each part of their business process, before moving onto the next stage of the program and enables employees to plan their study time around their current work commitments. The result is far greater program benefit, over a more sustainable period of time and a significantly improved return on investment.

Appleton Greene uses standard and bespoke corporate training programs as vessels to transfer business process improvement knowledge into the heart of our clients’ organizations. Each individual program focuses upon the implementation of a specific business process, which enables clients to easily quantify their return on investment. There are hundreds of established Appleton Greene corporate training products now available to clients within customer services, e-business, finance, globalization, human resources, information technology, legal, management, marketing and production. It does not matter whether a client’s employees are located within one office, or an unlimited number of international offices, we can still bring them together to learn and implement specific business processes collectively. Our approach to global localization enables us to provide clients with a truly international service with that all important personal touch. Appleton Greene corporate training programs can be provided virtually or locally and they are all unique in that they individually focus upon a specific business function. All (CLP) programs are implemented over a sustainable period of time, usually between 1-4 years, incorporating 12-48 monthly workshops and professional support is consistently provided during this time by qualified learning providers and where appropriate, by Accredited Consultants.
Executive summary

Acquisitive Growth
In today’s context of changing markets, technologies and business models, and in conjunction with historic levels of available capital, acquisitive growth has emerged as an increasingly compelling approach to transformational growth. However, as has been empirically proven growth through acquisitions is fraught with pitfalls and inherently risky. Successfully acquisitive growth requires the confluence of many factors that go beyond the traditional phased steps of a typical process. In my experience success is a function of bringing together the elements of people, processes, and technologies into a set of capabilities that are custom-made for an organization’s particular strengths, circumstances and aspirations. Winning in today’s dynamic markets demands bold, unique and sustainable strategies. The following are the stages of such an approach that I have found to create high probability, profitable growth that stands the test of time.
Additionally, while the M&A industry has many advisors available, they tend not to be operating executives who have lived through all the elements I will lay out below. Many simplistic guidelines exist, however what its clear is that the difference between success and failure with acquisitive growth is not in rote adherence to some set of processes, rather it is found in the combination of process discipline and strong application of experiential, practical knowhow. The nature of this knowhow is to apply and allocate the elements below in a smart, efficient manner to achieve exemplary outcomes for the specific client’s unique situation and circumstances.
Strategy Development: Whether at the corporate level or in a specific business unit, clients would be taken through steps to clarify the markets and segments where they currently compete and where they want to go in the future, what differentiates them from competition, where capabilities need to be refined or built, and the various functional elements (e.g. systems, processes, structures, etc.) critical to sustain profitable growth. Approach would be a combination of review of current strategies/capabilities, interviews and facilitated discussions and structured workshops. Outcomes might be a strategy to bring a particular business into a new growth phase or to meet changing competitive environments, or at the enterprise level might entail “platform building” whereby new businesses, sectors or legs are build from the ground up through foundational initial acquisitions and subsequent organic and inorganic initiatives.
Market Focus: Where will we hunt for acquisition targets? If a company allows too-wide of a scope will find themselves suffering from expensive resource drains/distractions and/or dilute efforts. Therefore, following the alignment of enterprise/business strategies the process will seek to focus the market segments and the business criteria to qualify a company to be elevated to possible target.
Research Possible Targets: Simply put, take the descriptions and criteria from above and create lists of potential targets that might fit. Each such company is researched for available information, any currently available knowledge the client might have, etc. Output is a gross list of possible targets.
Target Approach: Utilizing a number of possible approaches, one that is appropriate for the client is determined. For example, some companies may have business development or sales teams who could participate in this stage, or on the other hand for reasons such as confidentiality, resource scarcity, etc this might need to be put into the hands of specific individuals (senior executives, dedicated M&A executives, 3rd party services, etc.). Each company is different, so this is an exercise of matching needs with capabilities. The objective is to screen the gross target list to elminate those who have “killer facts” such as big contingent liabilities, prohibitive complexity such as a company with a complex ownership structure, our any other aspects that renders a target not acceptable for the next step.
Cultivation: This is a very critical part of the overall process. The essence of this authentic, genuine and meaningful relationship-buidling which requires a combination of individuals with certain skill-sets to ‘sell’ the prospects on being acquired, patience and persistence. I have many approaches, processes and techniques that I have and continue to use to great effect in this regard. Output is a short list of interested targets who have moved to active discussions and in-person meetings.
Target Assessment: During the cultivation phase as it gets more advanced, a critical success factor for effective acquisitive growth is the ability to narrow the list with limited amounts of information. This is important because the next phase is quite intensive so any company can not practically thoroughly assess all such targets. In other words, how does a client gain the insights needed to do this? Some might consider this the ‘phase I due diligence’ whereby, prior to the engagement of expensive resources such as lawyers, accountants, etc., an overview of a target’s current status is determined. Through structured and open discussions, the client engages in discussions with the targets to learn as much as possible..
Preliminary Offer: Structuring of a term sheet or letter of intent based on finding to date. Depending on these findings, certain terms may be included to lay out a) value expectations; b) focus for due diligences and commitment to support it; and c) various legal terms typical for these agreements. This tend to be non-binding agreements meant to establish exclusivity of dealings for a period of time, high level terms that both parties agree to, and confidentiality. Given my background, I have the abilility to craft these documents with minimal legal cost.
Due Diligence: This is yet another element of acquisitions that can take several different forms. Depending on the situation and capabilities of both clients and targets, due diligence activites tend to have different scopes and approaches that match each particular circumstance. A simple example would be a private company target versus a public company. With the latter, sellers often limit potential acquirers to only publicly available information whereas private companies may have limited information at their disposal. Therefore, each approach must be designed for purpose, with the output being a customized plan for a particular target. This leads to both more efficient and cost effective processes as well as deeper insights to help with final decisions.
Deal Making: After the due diligence phase, and with a set of terms already agreed, the negotiations begin to finalized the terms of value, liabilities and the myriad legal and busses considerations that must be addressed and finalized. Whether as chief negotiator or as a trusted advisor to the same, I would bring my experience and talent to bear on this phase as well as some structured approaches/guidelines.
Integration Planning: Concurrent with the commencement of due diligence, full attention is required to determine the structure, resources, plans and teams for post-closing integration. Specific approaches and processes would be employed here to ensure that a proper integration leader is named (critical), robust but prioritized integration plans (e.g. IT and Finance integration might be a first priority for some companies), organizational and assimilation plans, and specific actions in several other area. Among the more difficult and critical elements of integration is culture. While culture is a key consideration in the pre-offer phases, it tends to be among the more challenging aspects to successful acquisitions and an area where experience from a career of hands-on accountability of acquisitions brings valuable insights. Several pro-active approaches can be introduced to the clients to determine which is best to employ with any particular integration.
Execution: From plans to execution requires much more than a roadmap. While such roadmaps are critical, it is the confluence of leadership and human capital, prioritized focused actions to achieve specific results, and finally sustainable integration to bring into the client’s company the full potential of the value creation possible. Tools exist and can be created to provide structure and management support to achieve this consistently.

Important And Strategic Elements Of A Growth By Acquisition Approach
This program has thus far concentrated on the role that acquisition strategies play in driving growth.
However, this assumes that the acquisitions are carried out properly on its own. Experience has shown that acquisitions may both produce and destroy value, with the execution of the transaction typically making the difference.
The following are crucial and strategic elements that support successful acquisitions:
• Considering strategic fit: Purchasing merely for the sake of purchasing is little more than management hubris. The target businesses should in some manner meet the needs of the buyer’s company strategy (i.e. product or service line, geographic reach, etc.).
• Addressing culture fit: Due to cultural mismatches between the two merging organizations, some of the largest mergers in history have failed. It is important to take into account a company’s culture because it directly affects how it creates value.
• Doing thorough due diligence: This guarantees that the buyer “looks beneath the hood” of the company they are buying and that the price they are looking to pay for the company reflects its intrinsic value.
• Integration: Even when the share purchase agreement’s ink dries, the deal is not finalized. The two businesses must now start an integration process to ensure that they grow into something greater than the sum of their individual parts.

Advantages Of Growth Acquisition
10 advantages of expanding your company through acquisition
If you’re deciding whether to enter into an acquisition contract, you might wish to take into account the following list of acquisition benefits:
1. Strengthens a failing business
The company you work for might be going through a period of underperformance, and an acquisition might be the answer. The ability to work together as a team rather than alone may be a key factor in the business’ success. As you get to share resources with the company you’re merging with, this can assist keep the business from failing.
2. Secure financing for growth
By making an acquisition, a company might gain access to money or other important assets that it might not otherwise have at its disposal. You can easily acquire these assets with the aid of an acquisition. The firm and its employees may benefit from collaborating with a company that has sufficient resources because the development of the enterprise is the ultimate objective.
3. Have access to skilled personnel of high caliber
An acquisition can aid in boosting both the amount and quality of employees who are knowledgeable about the demands of the company. The experienced staff often stays on the firm payroll after an acquisition is completed so they can integrate. Their business acumen contributes to the companies’ success after the merger.
4. Expand the company’s market.
The corporation may diversify its offerings of goods and services as a result of the acquisition. You can make a variety of goods and distribute them to various target consumers. An acquisition often aids in a company’s development and growth.
5. Increase market influence
When you enter a new market, making an acquisition might help you combine market forces and exercise control. The synergy it offers increases your market presence and market share. If you plan to establish branches or subsidiary businesses, an acquisition may assist you lessen competition and preserve market dominance.
6. Make sure more capital is available.
Because the company is now larger after an acquisition, access to cash is improved. Higher cash and funds are available and accessible as a result of the arrangement. Amountable capital may be extended to both companies according on the agreement the companies come to when making the purchase.
7. A decrease in training expenses
Through an acquisition, your company may be able to cut internal training costs by using resources from the other acquired company. The cost of employee training is not necessary if the acquired firm develops its resources. You can use the company’s resources, depending on their state of development, to train other employees so they can develop their skill set.
8. Boost the competitiveness of your business
A purchase can take care of the requirement to adhere to higher standards as a result of the development in technical advancements. By joining forces with a smaller company that possesses the required technologies, a larger corporation can maintain its competitive position. Long-term gains from this may accrue to both businesses.
9. Lower production expenses
If you can use another company’s production facilities, facilities, and storage space, merging with them can save your production expenses. Building these kinds of facilities can be expensive, but if the business expands, it might be necessary. Sharing resources could significantly affect the budget and production costs.
10. Enable you to fulfill stakeholder expectations
Stakeholders could have expectations for the company’s growth, and making an acquisition is an effective strategy to achieve such expectations. An purchase increases the likelihood of investment returns, which may gratify the stakeholders. The pressure from the stakeholders can be handled more easily by making an acquisition, and you can even surpass their expectations.

What To Watch Out For During The Entire Acquisition Growth Process
Investigating less evident problems within the target company is the goal of the due diligence procedure.
This ranges from contracts with sizable clients that are about to expire to potential legal proceedings resulting from past business decisions.
But there are a few things that the buyer should watch out for on a more strategic level.
They consist of the following:
• Culture: Even if this phrase keeps coming up, it is crucial to the success of M&As. The culture of the target company should be thoroughly researched by prospective buyers in order to have a sense of what they are getting into.
• Competitive Edge: Is the target company “plain vanilla” or does it engage in any activities that offer it a competitive advantage (which we’ll define as the capacity to produce above-market value over the long term)?
• Leadership: Would the target company’s leadership complement your own leadership team in a positive way? Spend some time with them while conducting your research to see whether this might be the case.
• Possibilities: Are there any prospects that the target firm can take advantage of that your business won’t be able to in the near future? Let’s say it’s because of a service or product line they offer that is expected to see rapid expansion.
• Synergies: Where do your two companies’ synergies lie? Are they really complementary, or does purchasing the target company actually run the danger of causing some of your company’s income streams to be cannibalized?
Program Objectives
The following list represents the Key Program Objectives (KPO) for the Appleton Greene Acquisitive Growth corporate training program.
Acquisitive Growth – Part 1- Year 1
- Part 1 Month 1 Business Assessment – Assessments can be incredibly valuable tools for organizations of all sizes. A comprehensive assessment methodology can help you evaluate your organization across multiple dimensions. But what are business assessments, what do they entail, and what are the benefits? Business assessments can help you identify areas of improvement and potential acquisitive growth. By taking a comprehensive approach, you can get an accurate picture of your organization’s strengths and weaknesses. Assessments can also help you develop actionable plans to improve your business. At their core, business assessments are all about providing clarity. When you’re feeling overwhelmed by the day-to-day details of running a business, it can be difficult to step back and get a clear picture of where your company is headed. That’s where assessments come in. By taking a comprehensive look at your company’s strengths and weaknesses, you can develop a clear road map for success. Assessments are an essential part of any business plan. By evaluating your company’s strengths and weaknesses, you can develop a roadmap for growth. Furthermore, assessments can help identify areas where your company may be at risk. By addressing these risks early on, you can avoid potential problems down the road. In addition, assessments can help you benchmark your company’s performance against others in your industry. This benchmarking process can give you valuable insights into areas where your company may need to improve. Ultimately, regular business assessments are a crucial tool for any organization that is looking to grow and thrive.
- Part 1 Month 2 Strategic Aspiration – A Winning Aspiration defines the purpose of your enterprise, its guiding mission and aspiration, in strategic terms. The first choice of the strategic choice cascade is winning aspirations. Here we ask, “what is our winning aspiration.” Strategically, our winning aspiration defines our purpose. Aspirations are a view of the future. Qualified with “winning,” it is the ideal future that we strive to achieve. Unless you deliberately set out to win, it is impossible to do so. A business that only wants to participate rather than succeed will invariably fall short of making the difficult decisions and large investments necessary to succeed. Aspirations that are too modest rather than lofty are much more harmful. Most businesses fail because they have low expectations.
- Part 1 Month 3 Segment Focus – Every company aspires to grow. But, in a market where competition is fierce, inorganic business growth requires insight and innovation. Segmenting the market and customers is among the most effective techniques to promote acquisitive growth. Yet as numerous businesses have shown, artful segmentation can result in a significant competitive advantage. The purpose of segmentation is to inform your marketing approach. Using this method, it is feasible to recognize and categorize groups of potential clients based on their shared preferences, needs, and interests. This method effectively identifies the demographics most likely to value a specific good or service you provide. Furthermore, it may assist you in positioning that service so that it outperforms that of your rivals.
- Part 1 Month 4 Targeted Offerings – Everything the market offers, be it products or services or any experience, is known as a market offering. Market offerings are also divided among themselves based on the nature of the offering. Read along to understand the role and value of market offerings. Individuals within a market have different wants and needs. As a result, businesses in the market offer various products and services. The ultimate aim of businesses is to fulfill all the varying wants and needs of the population. Providing better target offerings and standing out in the market will eventually lead to more loyal customers and a broader customer base. People expect businesses to add value to their lives in various ways, precisely the purpose of market offerings – satisfying customer needs.
- Part 1 Month 5 Target Pool – The purpose of this workshop is to map out the offerings that one wants to develop or enhance for the focus segments defined by WDP3. A target pool is at the intersection of Targeted Offerings and Focused Segments. For example, if your strategy is focused on growing a currently manufactured product beyond your existing markets, you’ll want to know all the players who make these products in the markets where you don’t currently play but aspire to. In this simple case, the target pool would be derived by researching the current suppliers in these focus segments and profiling them for certain things such as size, channels to market, etc. The approach of this workshop is to take the Targeted Offerings and in a way and ‘map’ them with the Segment Focus areas we developed previously. In reality you might only need to do one or few of these approaches, but the workshop can develop the understanding and skills to do this work, which is in essence synthesizing the ‘strategic play’ associated with any acquisitive growth program.
- Part 1 Month 6 Target Identification – Target identification in acquisitive growth is the process of identifying potential companies or assets that align with the strategic objectives of the acquiring company. It involves conducting comprehensive research, market analysis, and due diligence to evaluate various factors such as financial performance, growth potential, synergies, industry trends, competitive landscape, and cultural fit. The goal is to identify targets that offer strategic value and can contribute to the acquirer’s growth, profitability, market position, or diversification objectives. This process requires careful evaluation, consideration of risks, and alignment with the acquiring company’s overall M&A strategy to ensure successful integration and value creation.
- Part 1 Month 7 Target Approach – All business investors are “financial” investors – the real question is how “strategic” is their ability to leverage the assets of the target. Providing practical guidance on approaching a business target and conducting initial due diligence depends on the investor’s criterion, competencies, and execution bandwidth. At this point, you will have identified a target or group of targets and you are attempting to learn enough about the target to determine whether to proceed with developing a meaningful indication of interest. Of course, an active seller is likely prepared for the sale process and represented by an advisor who is postured to provide the financial and operating information necessary for investors to quickly determine the suitability of a deal (i.e., a pitchbook and defined protocols for communication and information access). However, many desirable targets may not be seeking a sale because business conditions are favorable, and their businesses have been managed to provide options to the owners regarding continued independence and turn-key ownership and management succession. If the former, you, as a prospective buyer may have already pinged on the radar of the seller, and if the later, you have mined for target opportunities and are ready for the next step to accomplish an acquisition.
- Part 1 Month 8 Deal Approach – The M&A landscape is becoming increasingly competitive and the balance of power is shifting further in favour of buyers. For attractive businesses, however, sellers may wish to make divestments through an auction process which is designed to elicit competitive bidding among interested parties to facilitate the sale of a business or stake in a company at the highest price and on the best possible terms. Not all transactions require collaboration between the buyer and the seller, however. In many instances, an auction is still a better approach than a negotiation. The trick is in knowing which process to use when. To make that choice, you need to clearly understand your potential buyers, the characteristics of the asset in question, your own priorities, and the relative importance of speed and transparency to obtaining the best price.
- Part 1 Month 9 Cultivation – (non-auction)
- Part 1 Month 10 Cultivation – (organized process)
- Part 1 Month 11 Confirm Target – Assuming initial contact and conversations go well, the acquirer asks the target company to provide substantial information (current financials, etc.) that will enable the acquirer to further evaluate the target, both as a business on its own and as a suitable acquisition target. After producing several valuation models of the target company, the acquirer should have sufficient information to enable it to construct a reasonable offer; Once the initial offer has been presented, the two companies can negotiate terms in more detail.
- Part 1 Month 12 Talent Assessment – Talent decisions can be made with less precision, discipline, and data but frequently require more complexity than other integration decisions (such as decisions about goods, markets, or customers). M&A leaders must “up their game” in talent assessment if they want to succeed. In the end, the acquirer must decide if current employees from the target (the acquired company) are the most qualified to carry out the goals of the new organization.
Acquisitive Growth – Part 2- Year 2
- Part 2 Month 1 Talent Strategy – There are numerous tactics available for talent acquisition. But not every organization benefits from every method or strategy. When developing your strategy, consider the following factors: industry, size, development trajectory, types of positions, leadership, and more.
- Part 2 Month 2 Integration Strategy – The process of integrating a buyer and seller to the extent required to realize the anticipated benefits from a merger or acquisition is known as an M&A integration. An M&A integration plan outlines the merger’s goals, top priorities, performance indicators, non-negotiables, and scope.Getting agreement among your leaders on the integration strategy is the first stage in an M&A integration. At least two to three months before the deal closes, they should make it clear.
- Part 2 Month 3 Business Plan – Lack of a business strategy before an acquisition is one of the main mistakes that many M&A practitioners make. When considering an M&A, the business strategy is a vital resource. It provides comfort to those funding the deal that the reasoning behind it is sound and that the decision to acquire is not being made on a whim, as well as a roadmap for what you’re looking for in a business acquisition.
- Part 2 Month 4 First 90-Day Plan – HR must be quick and efficient when acquisition is at the core of a company’s growth strategy. The first 90 days are crucial for the organisation’s long-term performance as well as for the retention of individual employees. We can win hearts and minds by day 90 and have a better probability of them becoming productive team members if we have a robust acquisition plan.
- Part 2 Month 5 Valuation – One of the biggest challenges in negotiating a business acquisition is typically price haggling. The intricacy of business valuation makes this more challenging because a fair value cannot be determined without thoroughly examining the company’s financial data, sales trends, customer and supplier base, and many other factors.
- Part 2 Month 6 Synergy Analysis – A significant driver of value in M&A transactions is the potential for establishing synergies. A synergy is the idea that two businesses might be valued more highly when united than when valued separately. Knowing the possible synergies in an M&A transaction is crucial to any agreement, for both the buyer and the seller.
- Part 2 Month 7 Due Diligence (Foundational – Foundational due diligence is an organization’s baseline due diligence requirements that they must have on file for every vendor relationship, regardless of risk level, in order to do business with them. With origins in the private-sector world of business and finance, the term “due diligence” refers to the process through which an investor (or funder) researches an organization’s financial and organizational health to guide an investment (or grantmaking) decision. The decision to fund or not to fund is based upon a balance of objective data analysis, insight into the general state of organizational health and stability, and intuition. A sound and thorough due diligence review is the process through which all the factors that make up that equation are uncovered and understood. It is the process in which a program officer seeks the “truth” about an organization. Foundation program officers are faced with multiple challenges in assessing whether to recommend a grant to their board or decision-making committee. First, they must ascertain whether and to what extent the proposed activity coincides with the foundation’s guidelines and priorities. Next, they must assess the worth of the proposed activity itself — does it advance the fi eld, provide needed services or generate new learning? If the proposal survives this initial scrutiny, it must then be weighed for its relative merits beside many other worthy proposals. This process requires a great deal of skill and sensitivity. Due diligence protects a foundation’s investments and reputation and advances its mission and overall strategy.
- Part 2 Month 8 Due Diligence (Business Plan) – When you receive a proposal on your desk, the first step of proposal review is generally a consideration of the alignment of the applicant organization and proposed project with your foundation’s guidelines and interests. If this initial review is positive, due diligence typically commences with broad research and information gathering to provide a good understanding of the organization, how it fits into the field and the way in which this project will advance your foundation’s strategy. You might also contact colleagues for their view of the organization and its work. Then, you move on to get to know the applicant on a deeper level, including interviews with some combination of the executive director, board chair, other board members and staff members key to the proposed project. Each of these activities is covered in depth in this tool.
- Part 2 Month 9 Deal-Making (Direct Negotiation) – Direct negotiations are a deal making process in which an agency may contact a single contractor of its choice to submit a quote or tender without having first gone through a genuine competitive process.
A variation to an existing contract can also be a direct negotiation. Bargaining between buyer and contractor is a critical element of the process. The objective is to reach agreement on all terms and conditions and to obtain the goods and services at a price that is fair and reasonable to both the contractor and the agency. Direct negotiations are not intended to avoid competition or to discriminate against any organization and must be conducted in a manner consistent with the standards of behavior and requirements. A suitable assessment, based on comprehensive knowledge gained through specific market research, will need to be made to justify direct negotiation. - Part 2 Month 10 Deal-Making (Auctions) – Many (if not most) complex deals between buyers and sellers—from home sales to purchasing auctions to corporate mergers—qualify as auction deal making. Deal making (auctions) give sellers the opportunity to avoid making the difficult tradeoffs of traditional negotiations or auctions— competition versus value creation, for example, or many versus few bidders. In fact, sellers can take the best of both worlds— negotiations and auctions—to ensure they get a great deal. Auction deals have the following features: 1. One-on-one negotiations. At some stage of the deal making process, the seller engages one or more buyers in private discussions about the asset on the table. 2. One or more rounds of bidding. The seller also pits potential buyers against one another in an auction. 3. Several, but not too many, potential buyers. Deal making at auctions need enough parties to spark an auction but not so many that one-on-one negotiation would be difficult for the seller to manage. 4. Process ambiguity. In a traditional auction, the seller determines the process (whether there will be a single round of bidding or multiple rounds, for instance), and buyers are passive participants. In auction deal making, by contrast, the process is up for grabs. Buyers can try to shape the process to their advantage, as in the case of an auction contestant who approaches a seller about negotiating privately to move beyond the single issue of price. In general, whether you are the process setter or a bidder in an auction that has features of a negotiation, don’t assume that the rules are set in stone. Instead, change the game by thinking about how you can influence the rules, parties, and assets to your advantage.
- Part 2 Month 11 Documentation – The paperwork phase of a merger or acquisition is crucial. It might be regarded as the merger and acquisition process’s soul. With due diligence complete, parties make the final decisions on moving forward to execute the transaction. For legal teams, this comes with several responsibilities. Corporate or pre-clearance filings must be made in advance of the closing date. These include merger filings, amendments, ordering of good standings, or issuance of bring-down letters.
- Part 2 Month 12 Communications – An increase in M&A activity indicates a potential deal for entrepreneurs, business owners, and C-suite executives. In the event that a tempting deal is successful, it would be advisable to view an employee communication plan as a crucial component.
Methodology

Acquisitive Growth
It’s challenging to make this kind of acquisition successful. Seven fundamental operating principles are used by profitable corporate and financial purchasers, according to research. Almost all phases of the acquisition process, from the selection of candidates through post-merger management, are impacted by these ideas.
• Insist on cutting-edge operating tactics.
• If you can’t identify the leader, don’t make the deal.
• Provide top executives with significant incentives.
• Connect pay to variations in cash flow.
• Accelerate the rate of change.
• Encourage lively interactions between the board, managers, and owners.
• Employ the top acquirers.

Insist On Cutting-Edge Operating Tactics
High-profile leveraged buyouts like those of Duracell International, Uniroyal, and RJR Nabisco have garnered a lot of attention since the early 1980s. Prices, clever financial arrangements, and bargaining strategies have received a lot of attention. However, the other 2,200+ buyouts that took place during that time period and the fundamental changes in operational procedures that led to profitable outcomes for many of those businesses have received little attention. Although many observers think that LBO enterprises find hidden treasures in the market, more often than not, they only concentrate on enhancing operations.
Two acquisitions, Sunglass Hut International and Snapple Beverage Corporation, show that operating performance—rather than financial leverage, market timing, or industry selection—is the main driver of value creation in successful acquisitions.
Desai Capital focused on accelerating sales growth and developed a new strategy to achieve so when it acquired Sunglass Hut. By acquiring smaller stores in turn and introducing a new store model, Sunglass Hut has expanded from 150 locations to more than 800 since the initial acquisition in 1988. This growth has led to an astounding 37% yearly return. The business introduced a broad product selection rather than depending on two or three popular lines, replaced clerks with limited knowledge of sunglasses with educated customer-service specialists, and implemented a low-cost regional approach.
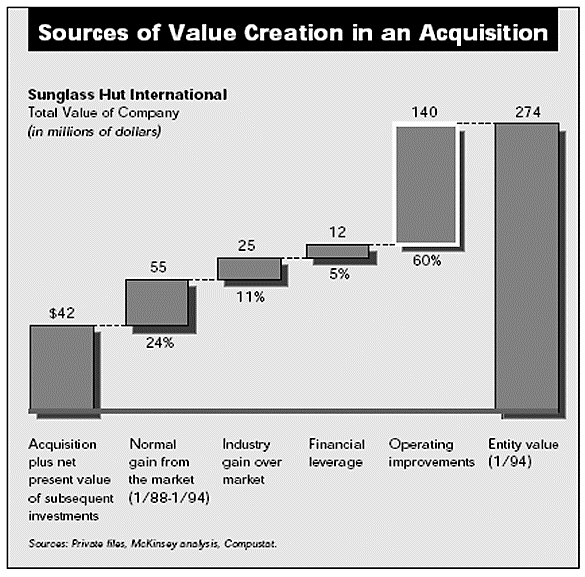
Another illustration of operating improvements is the 1992 purchase of Snapple by renowned financial acquirer Thomas H. Lee Company. Snapple launched an aggressive growth strategy based on quick global expansion and product range extensions shortly after the takeover. The business immediately established its production and distribution network since it anticipated that rivals will soon release their own natural teas and fruit juices. It entered into contractual agreements with bottling and distribution businesses that had excess production capacity, allowing it to launch its product one year before major rivals like Fruitopia (from the Minute Maid division of Coca-Cola Company) and achieve a competitive advantage.
As the Snapple case demonstrates, innovative operating methods help acquirers succeed in fiercely competitive markets like the American food and beverage sector. The takeaway: Don’t limit your search for success to high-growing industries.





